NUCLEAR TRACERS used in imaging prosthetic joints
The tracers discussed in this article are:
Tc-MDP: bone scintigraphy
Tc-sulesomab (trade name LeukoScan): in vivo labelled Fab fragment of IgG1. While this also binds to neutrophils (5%) and therefore should mimic WBC labelled scans it has a non-specific accumulation at infected sites. 35% of activity at 24hrs is in the bone marrow.
WBC labelled scans: in vitro labelled patient WBC with either Tc or In-111
FDG (Fludeoxyglucose 18F): is also used for infection imaging but PET scanning is not discussed in this article.
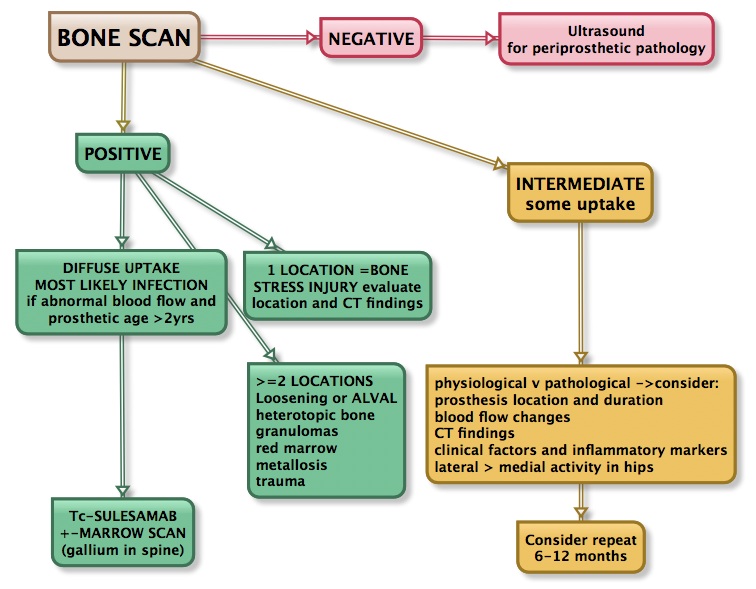
SUMMARY OF OUR APPROACH TO SCANNING PROSTHESIS
HIP PROSTHESIS
Normal Xray findings:
The stem should be in a neutral/anatomic position within the shaft and allow slight anteversion of the neck [1,2]. On an AP x-ray, the tip should be located in the center of the femur [1]. For a RA, the position of the resurfacing femoral stem should be central in the femoral neck on the frontal view (the position seen on the lateral view is thought to be less critical, but the pin should not impinge on the cortex) [1]. Occasionally there is notching of the superior femoral neck that is likely related to surgical trauma [1]. The recommended range for the acetabular cup inclination is 35-55 degrees (30-50 degrees [2]) and for cup anteversion 10-30 degrees [1] (other indicate 5-25 degrees [2]). A vertically oriented cup may result in excessive loading at the edge of the acetabular cup. The prosthetic femoral head should be symetrically seated within the acetabular prosthesis (or slightly inferior) . A femoral head located superiorly (even midly) is not normal and indicates polyethylene wear [2].
Normal scintigraphic findings:
Increased blood flow or focal blood pool abnormalities are infrequent in uncomplicated prostheses[3]. Diffusely increased blood pool activity within the muscle groups of the ipsilateral leg is seen in 20-40% of patients at some time during their post-operative course and can be seen up to 18 months post operatively. Delayed activity at the distal tip of the prosthesis can be seen in all porous prosthesis at some time. Even 2 years post-op, activity could still be identified medially (15%), distally (70%), and laterally (50%). Activity in the medial segment was always less than or equal to lateral segment activity.
Increased tracer activity can be seen aroundt the acetabular prosthesis in up to 76% of patients even after two years [4]. In uncomplicated prostheses, acetabular activity generally remains stable or decreases in intensity over time [4].
Persistently increased uptake of Tc-MDP in heterotopic bone occurs in most patients with hip prostheses.
A normal BONE scan essentially excludes a prosthetic complication [5].
Loosening:
Lucency around the prosthesis or migration of the prosthesis are the classical signs of loosening [1]. It is normal to identify a thin, linear lucent zone at the prosthesis cement interface, especially at the proximal lateral aspect of the stem [2]. This finding should be considered normal if stable, but any enlargement is suggestive of loosening [2]. Another common finding is a thin radiolucent band that is less than 2 mm thick and is demarcated by a sclerotic dense line running parallel to the stem along the bone-cement interface [2]. This band results from a reaction between the cement and the adjacent bone, with formation of a fibrous membrane [2]. A similar finding can be seen about cementless protheses, and should be considered normal if non-progressive after two years [2]. A “bone pedestal” is a transverse sclerotic line below the tip of the stem bridging the medullary canal [2]. Cortical thickening occuring in the femoral shaft at the level of the distal end of the stem result from stress alterations and reflect successful fixation of the stem [2].
Loosening is being increasingly recognized as the result of an immune reaction (type IV hypersensitivity) between the patient and the prosthesis [6]. Histopathologically, a pseudomembranous structure develops : Aseptic lymphocytic vasculitis-associated (or lymphocyte dominant) lesion (ALVAL) is the name that is now commonly applied to the condition [7]. It has also be referred to as aggressive granulomatosis and plain xrays are usually normal unlike aseptic osteolysis where plain film findings show a progressive lucent line wider than 2mm adjacent to 3 of the 7 regions about the prosthesis.
The granulomas can be identified on US, CT, and MR [1]. The lesions may have a variety of appearances and appear either cystic or solid
On bone scintigraphy, focal abnormalities (not diffuse) in two or more zones or intense uptake in one zone about the prosthesis is indicative of loosening but in uncemented prostheses an increased tracer activity can still be seen in some at the distal tip at 2 years: medially (15%), distally (70%), and laterally (50%). [8]. Generally focal uptake at one site may be considered normal bone stress if < 2yrs and abnormal bone stress or stress fracture at > 2yrs.
Gallium scintigraphy can demonstrate increased uptake in aseptic loosening which may be misinterpreted as infection. Gallium therefore has a doubtful role in assessing prosthetic joints. In-111 WBC or Tc sulesamab imaging should not be positive with aseptic loosening due to the absence of leukocytes.
Infection:
Combined In-111 WBC-bone scintigraphy imaging has a sensitivity of about 67%, and a specificity of 78% for the detection of prosthetic infection.Combined leukoscan (99mTc antigranulocyte antibody Fab’ fragments) and bone imaging had a sensitivity 85-92%, specificity 75-77%, positive predictive accuracy 58%, and negative predictive accuracy 93% for orthopaedic infection [10,11]. With infection, classically, the bone scan is 3-phase positive with diffusely increased periprosthetic activity on delayed images associated with infection (as opposed to loosening in which there is a focal abnormality identified about the prosthesis) [5,6]. The appearance is probably due to generalized osteolysis. Unfortunately, the findings on bone scan are non-specific and there is considerable overlap between infection and loosening [5]. False positive scans can occur due to dystrophic ossification, periprosthetic granulomas, the altered distribution of red marrow, aseptic loosening, damage of the polyethylene surface of the prosthesis, and metallosis.
The combination of either white cell or Tc-sulesamab with bone marrow imaging reduces the false positive scans and increases the diagnositic accuracy up to 90% [12].
Joint aspiration suffers from false positive (3-16%) and false negative (up to 10%) results [5,9]. Xray findings are normal in >50% of patients with a septic hip prosthesis. Non-focal periprosthetic bone resorption more than 2mm wide is non-specific and can also be seen in loosening.
Labeled WBC imaging: combined marrow scintigraphy using In-111 WBC’s and Tc-sulfur colloid provides the best results for the evaluation of the suspected infected prosthesis with sensitivity, specificity, and accuracy greater than 90% [5,6, 12,13,14]. Similar findings are reported with Tc-sulesamab combined with bonme marrow imaging, using time activity curves, or additional 24hr imaging [12]. Uptake should be absent in loosening (in which the associated inflammatory reaction lacks significant leukocytes) [6]. Normal WC or to a lesser extent Tc-sulesamab uptake is seen post joint replacement due to marrow uptake. Mild WBC accumulation can also be seen within heterotopic bone.
Any spatially incongruent area of In-111 WBC tracer activity which does not match marrow activity should be considered consistent with infection (regardless of the intensity of the tracer uptake) [5]. When the distributions of the two tracers are spatial
Sequential bone-gallium imaging can also be used to evaluate for prosthesis infection, but the overall accuracy (70-80%) is significantly less than that of combined In-111 WBC – Tc-Marrow scintigraphy [5,6]. For the gallium exam, the study is negative for infection when the gallium scan is normal, regardless of the bone scan findings, or when the spatial distributions of the two tracers are congruent and the intensity of gallium uptake is less than that of the bone tracer [5]. The exam is positive for infection when the distribution of the two tracers are spatial incongruent or when their distributions are spatial congruent, but the intensity of gallium uptake exceeds that of the bone agent [5]. The images are equivocal for infection when the distributions of the two tracers are spatial congruent and the intensity of uptakes are similar [5]. Unfortunately there are other non-infective causes of gallium uptake so a negative test is clinically useful but a positive test is not specific and requires considering additional factors such as tracer distribution, bone changes, and clinical findings.
Heterotopic bone formation:
Heterotopic new bone formation occurs in 15-50% of patients following hip replacement, but a clinically significant limitation of motion is rare (1-5%) [15]. Predisposing factors include male sex, total cemented prosthesis, lateral surgical approach, infection, post-traumatic arthritis, ankylosing spondylitis, and previous hip surgery [16]. Intense uptake is seen on bone scanning when the new bone is immature. Decreasing uptake can be seen as the bone matures. There is a lower recurrence rate of recurrent heterotopic bone formation if surgical removal of the bone undertaken after it has reached maturity.
KNEE PROSTHESIS
In general the principles for the knee are similar to the hip but it is more difficult to assess total knee replacements on bone scan, because 60% of the femoral prosthesis and 90% of the tibial prosthesiss demonstrate increased periprosthetic activity more than 1 year after surgery in asymptomatic patients [12,18] and periprosthetic activity can persist for several years [6].
The incidence of infection after TKA has ranged from 1 to 2% [17,5]. The risk for infection following revision is slightly higher (about 5%) [8]. Aspiration of the knee is helpful, but negative results do not exclude a deep infection [7].
White blood cell imaging has a sensitivity, specificity, and accuracy of approximately 85% in the diagnosis of infected knee prosthesis [16]. False positive exams have been reported in association with active rheumatoid arthritis and with massive osteolysis of the adjacent femur and tibia [16].
POST-OPERATIVE EVALUATION OF SPINAL FUSION
NORMAL FINDINGS:
- Generally, significant healing of the fusion is complete by 6 months, but as at other sites of surgery, scintigraphy may remain positive for up to 2 years. On SPECT images successful fusions demonstrate diffuse, but not increased, uptake which can be inhomogeneous, and no evidence of focal abnormalities within the fusion mass. This uptake is probably due to widespread new bone formation. Uptake within the vertebral bodies or apophyseal joints at the fusion levels may be seen and are more common with posterior fusions. These findings may be due to motion within the fusion, despite the absence of pseudoarthrosis. The activity seen within the fusion should decrease in size and intensity.
- Focal abnormalities within the spinal fusion mass have also been identified in asymptomatic patients, and may represent painless pseudoarthrosis. Late pseudoarthrosis (over 4 years old) may not demonstrate increased tracer localization and this may be related to decreased osteoblastic activity. These lesions, although present, may not be clinically significant as they do not alter skeletal metabolic activity. Due to the alterations in impact loading following fusion, focal uptake in the vertebral bodies and apophyseal joints in the free motion segments adjacent to the fusion are commonly seen in 45% of patients following lateral fusion and 87% of patients following posterior fusion (posterior fusion has been shown to produce greater stress in the free segments adjacent to the fusion).
- Asymmetric SI joint activity is also noted frequently in patients that have undergone spinal fusion (up to 75% of cases), with the side of the bone graft harvesting showing less activity than the contralateral side.
ABNORMAL FINDINGS
- Spinal fusion failure is characterised by reduced Tc-MDP uptake in the first 8-12 months and increased uptake after 18months
- The incidence of pseudoarthrosis following spinal fusion varies from 10 to 30%, however, not all patients with pseudoarthrosis suffer from back pain. Early failed fusion is usually treated with repeat surgery. Bone scanning can be used to detect the presence of a pseudoarthrosis in patients with continued pain following spinal fusion. A pronounced bilateral focal increase in Tc-MDP uptake within the fusion mass more than 1 year post-op is highly suspicious for pseudoarthrosis. Bone scintigraphy has a sensitivity of 78% and a specificity of 83% in the detection of pseudoarthrosis, which is superior to plain radiography (43% and 50%, respectively). [1,18,19,20,21]
- Increased Tc-MDP uptake is also seen associated with impingement from screws, adjacent segment pathology
- Increase SI joint uptake can also be seen and may be related to alterations in spinal mechanics [12].
- Infection in the spine is best identified using gallium rather than WCC [12] as false positive are rarely a problem. Whereas bone uptake is universal in prosthetic infection osteomyelitis of the spine can have little or no Tc-MDP uptake.
REFERENCES
- Ostlere S. How to image metal-on-metal prostheses and their complications. AJR 2011; 197: 558-567
- Mulcahy H, Chew FS. Current concepts of hip arthroplasty for radiologists: Part I, features and radiographic assessment. AJR 2012; 199: 559-569
- Oswald SJ, et al. Three-phase bone scan and indium white blood cell scintigraphy following porous coated hip arthroplasty: a prospective study of the prosthetic tip. J Nucl Med 1989; 30(8):1321-31.
- Oswald SG, et al. The acetabulum: A prospective study of three-phase bone and indium white blood cell scintigraphy following porous-coated hip arthroplasty. J Nucl Med 1990; 31: 274-280
- Love C, et al. Role of nuclear medicine in diagnosis of the infected joint replacement. Radiographics 2001; 21: 122901238
- Palestro CJ. Nuclear medicine, the painful prosthetic joint, and orthopedic infection. J Nucl Med 2003; 44: 927-929
- Watters TS, et al. Aseptic lymphocyte-dominated vasculitis associated lesion: a clinicopathologic review of an unrecognized cause of prosthetic failure. Am J Clin Pathol 2010; 134: 886-893
- Love C, et al. Diagnosing infection in the failed joint replacement: a comparison of coincidence detection 18F- FDG and111In-labeled leukocyte/ 99mTc-sulfur colloid marrow imaging. J Nucl Med 2004; 45: 1864-1871
- Squire MW, et al. Preoperative diagnosis of periprosthetic joint infection: role of aspiration. AJR 2011; 196: 875-879
- Ryan PJ. Leukoscan for orthopaedic imaging in clinical practice.Nucl Med Commun. 2002 Aug;23(8):707-14.
- Rugello D et al: Role of anti-granulocyte Fab’ fragment antibody scintigraphy (LeukoScan) in evaluating bone infection: acquisition protocol, interpretation criteria and clinical results. Nucl Med Commun. 2004 Jan;25(1):39-47.
- Palestro CJ. Radionuclide imaging of osteomyelitis. Semin Nucl Med 2015; 45: 32-46.
- Pelosi E, et al. 99mTc-HMPAO-leukocyte scintigraphy in patients with symptomatic total hip or knee arthroplasty: improved diagnostic accuracy by means of semiquantitative evaluation. Nucl Med 2004; 45: 438-444
- Palestro CJ, et al. Combined labeled leukocyte and technetium 99m sulfur colloid bone marrow imaging for diagnosing musculoskeltal infection. Radiographics 2006; 26: 859-870
- Mulcahy H, Chew FS. Current concepts of hip arthroplasty for radiologists: Part 2, revisions and complications. AJR 2012; 199: 570-580
- Clinical Orthopaedics and Related Research 1990; Rand JA, Brown ML. The value of indium 111 leukocyte scanning in the evaluaiton of painful or infected total knee arthroplasties. 259: 179-182
- Palestro CJ. Musculoskeletal infection. Ed. Freeman LM. Raven Press, Ltd. New York. Nuclear Medicine Annual 1994, 91-119
- Collier BD, et al. Bone scintigraphy: Part 2. Orthopedic bone scanning. J Nucl Med 1993; 34(12):2241-6. Review.
- Lusins JO, et al. Bone SPECT in patients with persistent back pain after lumbar spine surgery. J Nucl Med 1989; 30: 490-96
- Even-Sapir E, et al. Assessment of painful late effects of lumbar spinal fusion with SPECT. J Nucl Med 1994; 35(3):416-22.
- Slizofski WJ, et al. Painful pseudarthrosis following lumbar spinal fusion: detection by combined SPECT and planar bone scintigraphy. Skeletal Radiol 1987; 16(2):136-41